Research at Northwestern Qatar
Northwestern University is committed to protecting the rights and welfare of human research participants, per United States 45 CFR Part 46 Protection of Human Subjects, relevant ethical principles, and Northwestern University policy. Northwestern has an executed Master Agreement with Georgetown University in Qatar’s (GU-Q) Institutional Review Board (IRB) for the review and oversight of human research activities conducted at Northwestern Qatar.
Human Research Process Adaptation
As of January 1, 2024, an adapted process will be required of all individuals involved in the conduct of human research at Northwestern Qatar. A key piece of this adaptation is utilization of eIRB+. eIRB+ is Northwestern University's electronic system for all human research. In the months leading up, the Northwestern Qatar Research Office will provide resources and training opportunities to support compliance with these mandated practices.
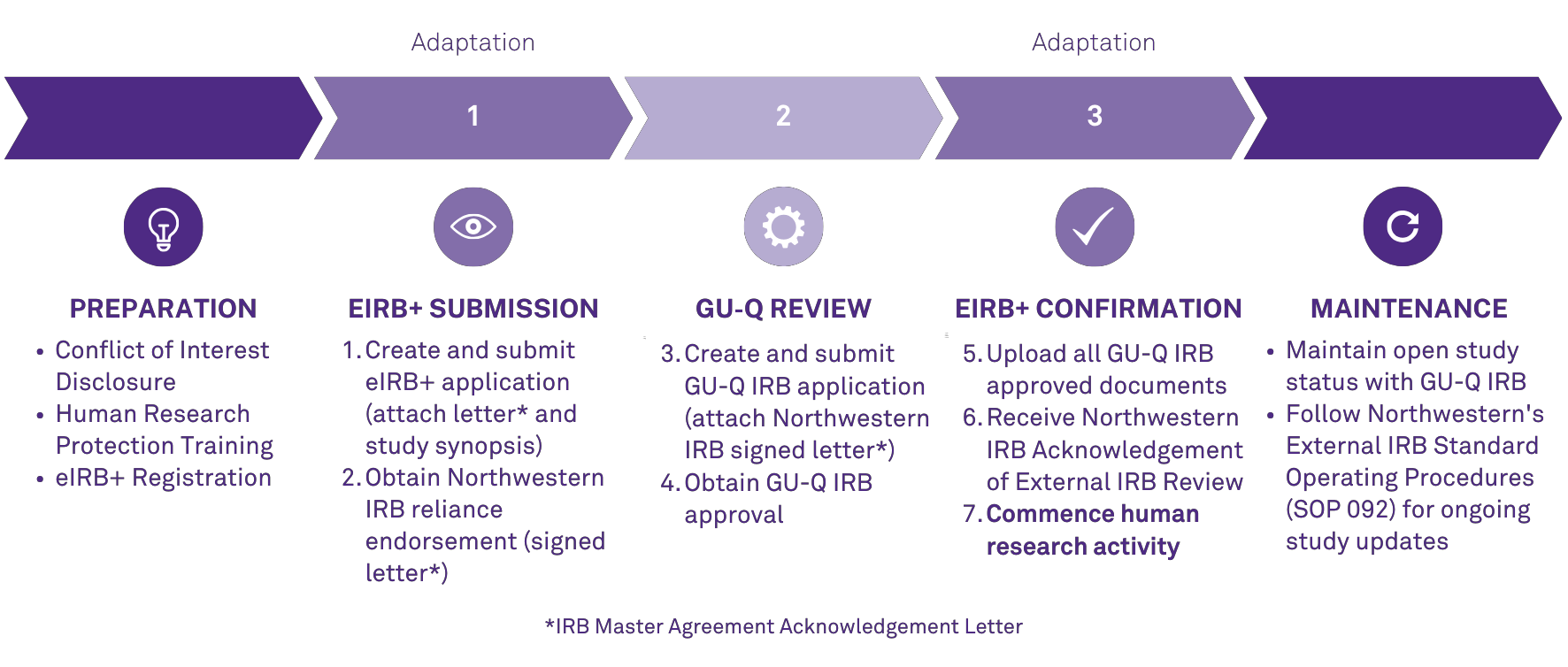
Preparation
- Conflict of Interest (COI) Disclosure: COI reporting is required annually through eDisclosure according to Northwestern University policy.
- Human Research Protections Training: Human Research Protections Training is required of all individuals engaged in the conduct of human research at Northwestern University prior to beginning human research activity.
- eIRB+ Registration: eIRB+ is the electronic system that houses researcher training records and human research study applications for the Northwestern University IRB Office. All individuals engaged in the conduct of human research must register in the eIRB+ system.
eIRB+ Submission
- Open eIRB+.
- Create an submission in eIRB+ following the "NU-Q Tutorial for Submitting a New Study Submission in eIRB+", linked under Resources.
- Enter study-relevant information into the "GU-Q IRB Institutional Clearance Letter" and include it with the eIRB+ submission. This document is used per study to simplify the reliance process because Northwestern has an executed Master Reliance Agreement with Georgetown University in Qatar’s (GU-Q) Institutional Review Board (IRB).
- Include a study synopsis with the eIRB+ submission; this may be the protocol you plan to submit to GU-Q or a grant proposal summary. The synopsis must detail key human research activities including study population(s), intervention(s), and objectives.
- All study team members must be registered in eIRB+, and included on the Study Team Members page under "Internal Study Team Members".
- After all information and required documents are included in the submission, the Principal Investigator (PI) must submit in eIRB+.
- Once submitted, the Northwestern IRB Office will review the submission, sign the GU-Q IRB Institutional Clearance Letter, and return the signed letter with feedback via eIRB+.
GU-Q Review
- Create and submit a GU-Q IRB application in MedStar (GU-Q's online system)
- Follow GU-Q processes and utilize GU-Q required templates
- Attach signed GU-Q IRB Institutional Clearance Letter to GU-Q application
- GU-Q IRB conducts IRB review of human research activities conducted at Northwestern Qatar.
- GU-Q IRB issues IRB approval
- Download all approved documents and GU-Q approval letter to next upload in eIRB+.
eIRB+ Confirmation
- Open eIRB+ and navigate back to your submission.
- Upload all GU-Q approved documents and the GU-Q IRB approval letter in eIRB+, following the "NU-Q Tutorial for Submitting a New Study Submission in eIRB+", linked under Resources.
- Resubmit in eIRB+.
- Northwestern IRB Office will review the submission and if all institutional requirements are met an Acknowledgement of External IRB Review will be issued via eIRB+.
- Human research activity may now begin at NU-Q.
Maintenance
- Ensure all GU-Q requirements are met to maintain open study status with GU-Q IRB.
- Follow Northwestern’s External IRB Standard Operating Procedures (SOP 092) for guidance on which study updates must be submitted in eIRB+, once IRB approved by GU-Q.
Resources
Georgetown University in Qatar (GU-Q) IRB
All human subject research conducted at NU-Q must be prospectively reviewed and approved by an IRB. The GU-Q IRB is responsible for the review of and oversight of the conduct of human subject research that is conducted at NU-Q per our master agreement. The GU-Q IRB performs both prospective and continuing reviews of every study involving research with human subjects. These IRB reviews are done in compliance with GU and Qatar’s Ministry of Public Health (MoPH) regulations.
No human subject research may be initiated, or continued, at NU-Q without prospective approval by GU-Q IRB and acknowledgement by Northwestern IRB. Investigators at NU-Q are subject to Northwestern and GU-Q requirements.
GU-Q Templates & Resources
MedStar IRB System
GU-Q uses the MedStar IRB System to manage their IRB review processes.
Frequently Asked Questions
How long is the Northwestern review process?
The NU IRB understands that all research is important and all research submissions are reviewed in the order received. We are paying very close attention to assure the research and research team uphold the core principles of our Human Research Protection Program (HRPP) and also honor our commitments to our partners.
At this time the approximate timeline for processing of a GU-Q Clearance Letter is 1-2 weeks from the time of assignment (not PI submission). This timeline my vary during periods of high submission volume and over US holidays. It also greatly depends on the completeness of the application.
One of the most significant ways to ensure all appropriate documents are provided at the time of submission. Change requests can add additional time to the review process. Submitting complete, detailed, and compliant IRB applications are the best way to reduce turnaround time.
Where do I submit my research study?
Step 1 of the Human Research Process Adaptation involves creating and submitting an application in the Northwestern electronic IRB system (eIRB+). Once Northwestern signs the GU-Q IRB Institutional Clearance Letter you can proceed to Step 2. Step 2 involves submitting in the GU-Q IRB electronic system (MedStar)
You are encouraged to review the information on this webpage and follow "NU-Q Tutorial for Submitting a New Study Submission in eIRB+", linked under Resources.
Contact Us
