Northwestern Relying on an External IRB
Reliance Key Terms
- Reliance Agreement - agreement between two IRBs that gives one entity’s/institution’s IRB the purview to review another entity's/site’s human research activities. Reliance agreements come in multiple formats, including Institutional Authorization Agreements (IAA), Memorandum of Understanding (MOU), and Master Reliance Agreement (MRA).
- Rely/Cede Review - The process of allowing another IRB (i.e., an External IRB) to review the research activities occurring at Northwestern.
- Relying Site - The site ceding review.
- IRB of Record - The IRB that is conducting the IRB review of a study.
|
External IRB Study Start-Up
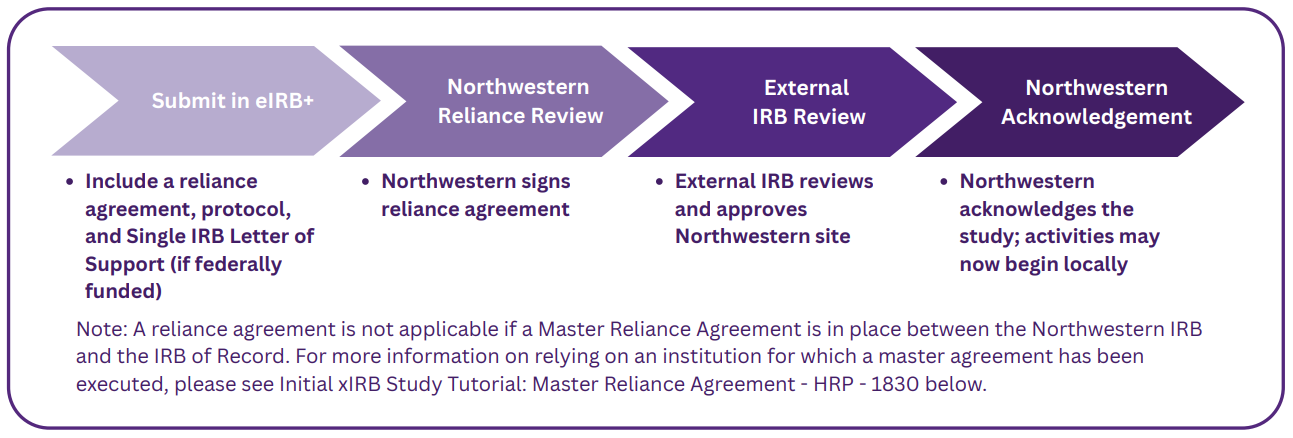
The following document provides a more in-depth outline of the steps and key benchmarks of the External IRB process.
The following is a step-by-step tutorial for the initial eIRB+ submission. This tutorial is applicable when Northwestern cedes review to an external IRB using a study-specific reliance agreement (e.g., IRB Authorization Agreement (IAA), SMART IRB Letter of Acknowledgement (LOA)), or when ceding review to Advarra or WCG.
The following is a step-by-step tutorial for the initial eIRB+ submission. This tutorial is applicable when Northwestern will cede IRB review to entities with which the Northwestern IRB has executed a master reliance agreement.
Consent Form Customization Tools
The following Consent Form Customization Tools denote Northwestern’s site-specific language requirements for consent forms for external IRB studies. These documents are intended to be educational and should not be utilized for studies where Northwestern will conduct a full IRB review of the research.
Reliance Agreement Templates
When ceding review to an External IRB, the External IRB will often prefer to use their own reliance agreement template. However, if the External IRB does not provide a reliance agreement template, either of the following templates may be used:
Commercial IRBs such as WCG or Advarra
Northwestern frequently cedes review to commercial IRBs. Our most commonly used commercial IRBs are Advarra and WCG. Northwestern has executed a Master Services Agreement with both Advarra and WCG. However, a study specific sign-off (i.e., Master Agreement Acknowledgement Letter) is still required to be executed prior to Advarra or WCG’s review of the Northwestern site. The following templates should be used when ceding review to Advarra or WCG in lieu of another reliance mechanism.
- If utilizing a commercial IRB other than Advarra or WCG, an IRB Authorization Agreement (IAA) or another type of reliance agreement will need to be executed on a per study basis.
If you would like Advarra or WCG to serve as the IRB of Record for your study and you do not already have a sponsor facilitating the use of Advarra or WCG, please utilize the instructions provided in the following budget estimate tools to contact the representatives from the respective IRBs to inquire about use of their services.
- Advarra Budget Estimate Tool (coming soon)
- WCG Budget Estimate Tool (coming soon)
Note: Industry sponsored studies are subject IRB Fees, charged by Northwestern. Please see our IRB Fees webpage for more information.
Institutions and Consortium Studies with Master Reliance Agreements
Northwestern has executed Master Reliance Agreements with the following institutions and consortium studies (not exhaustive):
Institutions and Consortium Studies with Master Reliance Agreements
| Institutions |
Consortium Studies |
|
Lurie Children's Hospital
National Cancer Institute's CIRB
|
ALL ALS - Mass General Brigham Hospital IRB
NeuroNext - Mass General Brigham Hospital IRB
Neals Network - Mass General Brigham Hospital IRB
StrokeNet - University of Cincinnati IRB
|
External IRB Study Maintenance
The eIRB+ study record must be kept up to date throughout the life of the study. Study team member updates and IRB of record approved study updates relevant to the Northwestern site must be submitted to eIRB+ via a modification. The following tutorial outlines how to submit modifications, continuing reviews, site/study closure, and other study updates for Northwestern IRB acknowledgement.
Other Tools and Guidance
The following document provides an outline of where documents should be uploaded across the IRBSITE and STU in eIRB+ and is applicable for both initial and modification submissions.
There likely will be additional documents that should be uploaded to the eIRB+ submission that are not specifically mentioned within this resource. If a document is specifically and only used at Northwestern, it should be uploaded to the IRBSITE. If a document will be used at or pertains to all sites (not including post-initial approval letters), it should be uploaded to the STU.
Reportable New Information (RNI) for External IRB Studies
Principal Investigator must submit reportable events to the External IRB per the External IRB’s reporting criteria.
If the reportable event occurs at or impacts the participants of Northwestern University and/or its affiliate sites (e.g., SRALab, NMHC locations), the Northwestern PI must report this information in parallel to the Northwestern IRB via an RNI submission in eIRB+, following Northwestern's Reporting Timeline Requirements, as described on the Reportable New Information (RNI) webpage under "Key Things to Consider".
Events (e.g., Unanticipated Problems Involving Risks to Subjects or Others (UPIRSOs), Adverse Events, etc.) that do not involve Northwestern University or its affiliates’ study participants are not required to be submitted to the Northwestern University IRB via a parallel eIRB+ RNI submission.
If protocol changes occur following the reporting and/or resolution of an event not involving Northwestern University and/or its affiliate sites, and those changes impact Northwestern University and/or its affiliate(s), those changes should be submitted as a modification submission in eIRB+.
External IRB FAQs
Study Start-Up Questions
Will Northwestern cede review to an External IRB?
Generally, yes. If your study is non-exempt human research (i.e., expedited or full board), the Northwestern IRB, typically, is amenable to ceding review. For the Northwestern IRB to rely on an external IRB, a new study submission must be made in eIRB+. To begin the process of requesting Northwestern cede review, minimally, a protocol and draft reliance agreement (or indication that SMART IRB’s Online Reliance System will be used), must be included with your submission. We do not sign reliance agreements outside of eIRB+.
- If you are requesting Northwestern cede review to an institution with which we have executed a master agreement, a drafted study specific reliance agreement should not be included in your submission. More information on requesting reliance on an institution with which we’ve executed a master agreement can be found below.
- When ceding review to an external IRB, investigators should be familiar with the Northwestern IRB’s Standard Operating Procedures outlined in HRP-092 – SOP External IRBs.
- If the Northwestern IRB is currently the IRB for the Northwestern activities and you intend to transition to an External IRB, please contact us at irbreliance@northwestern.edu.
What if my study is federally funded?
If your study is federally funded, reviewed as expedited or full board, and engages more than one site in human research, a Single IRB (sIRB) may be federally mandated. A Single IRB is the selected IRB of Record that conducts the ethical review for each site participating in cooperative or multi-site research. Please see our Single IRB Planning webpage for more information on additional requirements that may be applicable such as obtaining a Single IRB Letter of Support. How do I request the Northwestern IRB to cede review to an external IRB?
To request the Northwestern IRB to cede review to an external IRB, a new external IRB submission will need to be made in eIRB+ following the applicable new study submission eIRB+ tutorial above (link).
How do I get a reliance agreement signed?
A reliance agreement must be included in your new external IRB eIRB+ submission. Your assigned Reliance Analyst will facilitate obtaining Northwestern’s signature on the document. Reliance agreements are not executed via email or any other avenue outside of eIRB+.
What do I do if I can’t find the intended external IRB in the list of External IRBs on the STU (i.e., under Q1 of the External IRB page of the STU).
Please reach out to irbreliance@northwestern.edu. We will search our system to verify whether that IRB is included in our system. If it is, we will tell you exactly how it is listed in our system so you can find and select it. If it is not, we will work with you to have that IRB added to our system. How do I customize a study-wide consent form for use at the Northwestern or SRA Lab site(s)?
- The study-wide or sponsor template consent form must be customized to ensure it meets the University’s local language requirements. Northwestern’s required language can be found in our consent form templates. Local language requirements apply to the following aspects of the consent form:
- Conflict of Interest Disclosures – Applicable when identified by NU COI
- Participant Cost – Applicable for clinical trials
- Subject Injury Language – Applicable for greater than minimal risk studies
- HIPAA – See our HIPAA, PHI, & PII webpage for more information on when HIPAA applies
When can I start research activities at Northwestern?
Once a new study receives initial acknowledgement from the Northwestern IRB, research activities may begin at Northwestern, NMHC, and/or SRALab. Personnel listed as internal study team members in eIRB+ may also begin research activities at this time. Please see our External IRB SOP for more information.
How long will an External IRB take to review and approve my study?
When a study is reviewed by an external IRB, that review is conducted by that IRB's representatives. They operate independently of Northwestern and their review processes and timelines are outside of our control. However, before the external IRB can review and approve research at Northwestern a reliance agreement must be in place. Most studies will require a signed reliance agreement; however, in some cases, a master reliance agreement may already exist.
- If a study-specific reliance agreement is needed, please review the Initial xIRB Study Tutorial: Signed Reliance Agreement Required – HRP – 1829.
- If you believe your study may fall under an existing master agreement, please refer to the Initial xIRB Study Tutorial: Master Reliance Agreement – HRP – 1830.
Study Maintenance Questions
Once modifications are approved by the external IRB, can they be implemented immediately, or do I need to wait for Northwestern to review and acknowledge the modifications?
Once a study receives initial acknowledgement from the Northwestern IRB, most subsequent study updates can and should be implemented once approval from the external IRB is secured. However, these study updates must still be submitted in eIRB+ to ensure our local study record is accurate and up to date. Please note, updates to consent forms, protocol, PIs, continuing reviews and site/study closures are expected to be submitted for acknowledgement within two weeks of receipt of approval from the external IRB.
The following study updates must be submitted for Northwestern review and have received Northwestern IRB acknowledgement prior to initiation of study activities or implementation:
- Updates to Co-Investigators or Key Personnel
- Updates to study documents not otherwise reviewed by the External IRB (e.g., when the Northwestern IRB is still serving as the privacy board and must review/approve HIPAA language).
Do continuing review and study/site closure approvals need to be submitted to the Northwestern IRB?
the Principal Investigator is required to ensure the eIRB+ submission accurately reflects the current approval status of the study. Continuing review and study/site closure approvals must be submitted to eIRB+ via a modification for Northwestern IRB acknowledgement within two weeks of receipt from the IRB of record.
Other Questions
Which IRB am I required to submit Reportable Events to if a reportable event or protocol deviation occurs?
Principal Investigator must submit reportable events to the External IRB per the External IRB’s reporting criteria.
If the reportable event occurs at or impacts the participants of Northwestern University and/or its affiliate sites (e.g., SRALab, NMHC locations), the Northwestern PI must report this information in parallel to the Northwestern IRB via an RNI submission in eIRB+, following Northwestern's Reporting Timeline Requirements, as described on the Reportable New Information (RNI) webpage under "Key Things to Consider".
Events (e.g., Unanticipated Problems Involving Risks to Subjects or Others (UPIRSOs), Adverse Events, etc.) that do not involve Northwestern University or its affiliates’ study participants are not required to be submitted to the Northwestern University IRB via a parallel eIRB+ RNI submission.
If protocol changes occur following the reporting and/or resolution of an event not involving Northwestern University and/or its affiliate sites, and those changes impact Northwestern University and/or its affiliate(s), those changes should be submitted as a modification submission in eIRB+.
What is the Northwestern IRB’s continued role in this research if we have ceded review to an external IRB?
After facilitating the execution of Reliance on an external IRB the Northwestern IRB maintains several responsibilities as a relying institution. These responsibilities include, but are not limited to ensuring…
- All personnel are qualified to conduct research (e.g., study team members have up to date CITI training, identified PI is eligible to serve as PI)
- The Northwestern consent form has been customized to contain all required local language
- State laws and regulations and Institutional policies are followed
I have been asked to link CERES with the IRB submission. What does this mean and how do I accomplish this?
Sponsored Research utilizes information from eIRB+ to complete their necessary workflows. For information to successfully transfer from eIRB+ to CERES, the eIRB+ and CERES records for your study need to be linked. This is accomplished by utilizing the SR Chooser on the IRBSITE and STU funding pages of the eIRB+ submission. To accomplish this, navigate to the funding sources pages, and click the existing funding row item(s) to open the pop-out. Or, if no funding is currently listed, click the [+Add] button to create a new row item. In the pop-out please carefully review Q#1 and the "NOTE" text. All funding from external sources should select "Yes". Once, "Yes" is indicated under Q#1, click the [...] button in the "CERES ID" field that appears. You will then see a pop-up where you can select the funding associated with the study.
