eIRB+ Compliance Workspace
In 2025, the IRB Office Compliance Team began conducting post-approval monitoring (PAM) in the IRB’s electronic submission system, eIRB+. The eIRB+ Compliance Workspace is within eIRB+ but separate from the IRB review activities performed in eIRB+. This workspace will have some familiar and new functionalities for working within eIRB+.
- See the Training Materials section for detailed step-by-step tutorials on completing a post-approval monitoring assessment in the eIRB+ Compliance Workspace.
- The Getting Started section outlines how to login and navigate the eIRB+ Compliance Workspace.
- The Granting Access to the Compliance Activity section has information about the Principal Investigator to granting access to the Compliance Activity.
- See the Completing the Compliance Activity section for how to complete the activity and communicate within the system.
- The Closeout of the Compliance Activity section outlines how to save the required documentation from the system.
- The FAQ section has answers to many common questions about the eIRB+ Compliance Workspace.
Training Materials
 eIRB+ Tutorial: Getting Started with the Compliance Workspace (HRP-2025)
eIRB+ Tutorial: Getting Started with the Compliance Workspace (HRP-2025)
eIRB+ Tutorial: Self-Assessment for the Research Community (HRP-2026)
eIRB+ Tutorial: Corrective and Preventive Actions (CAPA) for the Research Community (HRP-2027)
Getting Started
Find information in the sections below on how to log into the eIRB+ Compliance Workspace, navigate and communicate in the system, how to complete a PAM assessment, and saving the required documentation.
Logging into eIRB+
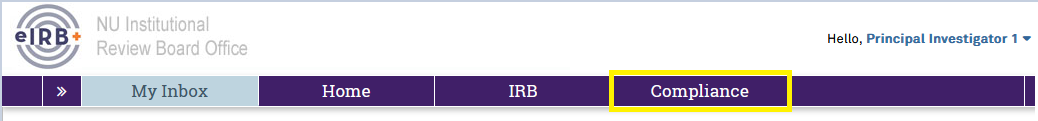
Researchers can access PAM activities, separate from IRB review activities, by logging into eIRB+ through the link on the eIRB+ Page and navigating to the eIRB+ Compliance Workspace on the purple navigation bar at the top of your screen, as shown in the screenshots below.
Navigating to the eIRB+ Compliance Workspace
Select the Compliance button on the purple navigation bar to access the eIRB+ Compliance Workspace.
The Compliance Workspace is divided into 4 tabs. If you are familiar with these tabs in eIRB+, you can jump right into the eIRB+ compliance workspace because the tabs work the same way.![]()
- Inbox shows the current compliance activities that require action from you.
- My Reviews shows all current compliance activities. This includes activities that require action from either you or the Compliance Team.
- Archived shows completed compliance activities.
- All Reviews shows all current and archived compliance activities.
Opening the Compliance Activity
Compliance Activities are identified by a unique 8-number code that differs from your study’s STU#.
This ID number begins with “COM.” For example, COM00000123. Select the ID number of the compliance activity located in the ID column to open the compliance activity submission. 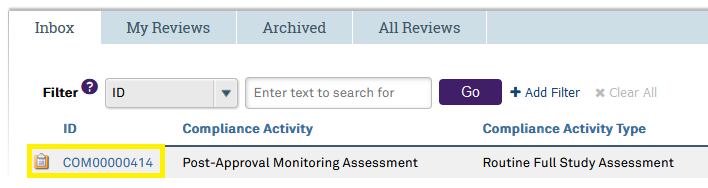
Granting Access to the Compliance Activity
Initially, only the Principal Investigator (PI) and the Primary Contact listed for the study can access the Compliance Workspace, receive notifications, and hold submission rights.
If someone else needs access to assist the PI in completing the Compliance Activity, the PI must grant them PI Proxy status. Please note:
- PI Proxy status allows individuals to access, receive notifications, and edit and submit responses within the Compliance Workspace
- Existing PI Proxies can grant PI Proxy status to other eIRB+ registered individuals
- Any access or submission rights for the compliance activity do not carry over to another study or compliance activity
See the eIRB+ Tutorial: Getting Started with the Compliance Workspace's Giving Study Team Access (PI Proxy Status) section for detailed steps.
Completing the Compliance Activity
Navigation within the activity has both familiar actions you may see when completing IRB submissions and new actions unique to the Compliance Workspace.
Open the compliance activity and click View/Edit Checklist to begin completing the PAM checklists. ![]() Respond to each item in the checklist(s) and follow the instructional text. Once you save your responses in the checklist(s), click Exit or Continue to exit the checklist. This will take you back to your compliance activity landing page.
Respond to each item in the checklist(s) and follow the instructional text. Once you save your responses in the checklist(s), click Exit or Continue to exit the checklist. This will take you back to your compliance activity landing page.
The Compliance Activity will no longer be in your Compliance Inbox. Instead, it will move to the Compliance My Reviews or All Reviews tabs. This will end the Pre-Submission state, and the Compliance Activity will enter the Pre-Review State.
The Compliance Analyst will review your responses with the eIRB+ Compliance Workspace and send the activity back to you with questions and required actions or send a closure notification if there are no observations or actions for you to take.
See the eIRB+ Tutorial: Self-Assessment for the Research Community's Completing the PAM Assessment Checklists section for step-by-step instructions on completing the checklists and responding to observations.
Communication during the Compliance Activity
Communication for observations and follow-up actions will occur primarily within eIRB+, similar to IRB submissions. eIRB+ will send system notifications as an email to the PI and all PI Proxies assigned to the Compliance Activity when their action is required.
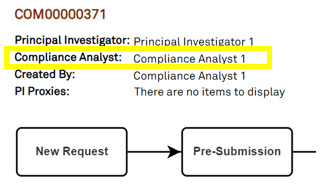
To find the assigned Compliance Analyst, open your compliance activity. Below the COM ID, the assigned Compliance Analyst is listed along with the Principal Investigator and PI Proxies:
You can contact the assigned Compliance Analyst in eIRB+ using the Add Comments icon ![]() under the Next Steps window on the left side of your Compliance Activity page. You may also email the assigned Compliance Analyst; see the Contact Us Page.
under the Next Steps window on the left side of your Compliance Activity page. You may also email the assigned Compliance Analyst; see the Contact Us Page.
Closeout of the Compliance Activity
The compliance activity is not complete until the activity is in a closed state. When the activity is officially closed, the PI and all PI Proxies will receive an eIRB+ system email notification with a closeout letter attached.
Print or save the electronic copies of the compliance activity and add to your study’s research records. Required documentation includes but is not limited to:
- Notification letter
- Closure letter
- Print review page of the activity
- Communication with the IRB Office
eIRB+ does not serve as an electronic version of your study files. The regulatory binder (where you keep all documents related to your study) should be centralized and can be maintained within an electronic format (saved PDFs and Word/Excel documents) or within a binder (printed paper copies). See our Study Support Resources and Templates Page for the Regulatory Binder and Research Record templates.
Frequently Asked Questions
Find answers to many common questions about the eIRB+ Compliance Workspace!
- See the eIRB+ Page for details on registering and logging into eIRB+.
- Review the Post-Approval Monitoring Page for more details on the PAM process and the various PAM Checklists.
- Review the PAM checklists on the Checklists & Worksheets
- Training Materials