December 2023 IRB Bulletin: News from the IRB Office
 UPCOMING IRB Brown Bag
UPCOMING IRB Brown Bag
Join us at Noon on January 17th for a special Brown Bag on consenting human participants in research where we will address updates to the consent template, review the key information section, discuss consenting for social behavioral research studies, and more.
ANNOUNCEMENTS
Highlighting Northwestern Office for Research Nationally
Northwestern University IRB Office staff presented a poster titled “A Novel Program to Support Researchers in Making Informed Incident Reporting Determinations” at the 2023 Public Responsibility in Medicine and Research (PRIM&R) Annual Conference in Washington, D.C. They presented data on their initiative to support the research community and IRB Office with research events that occur, which included releasing the Incident Assessment Tool (IAT) (HRP-1207) on the IRB Office website in June 2022.
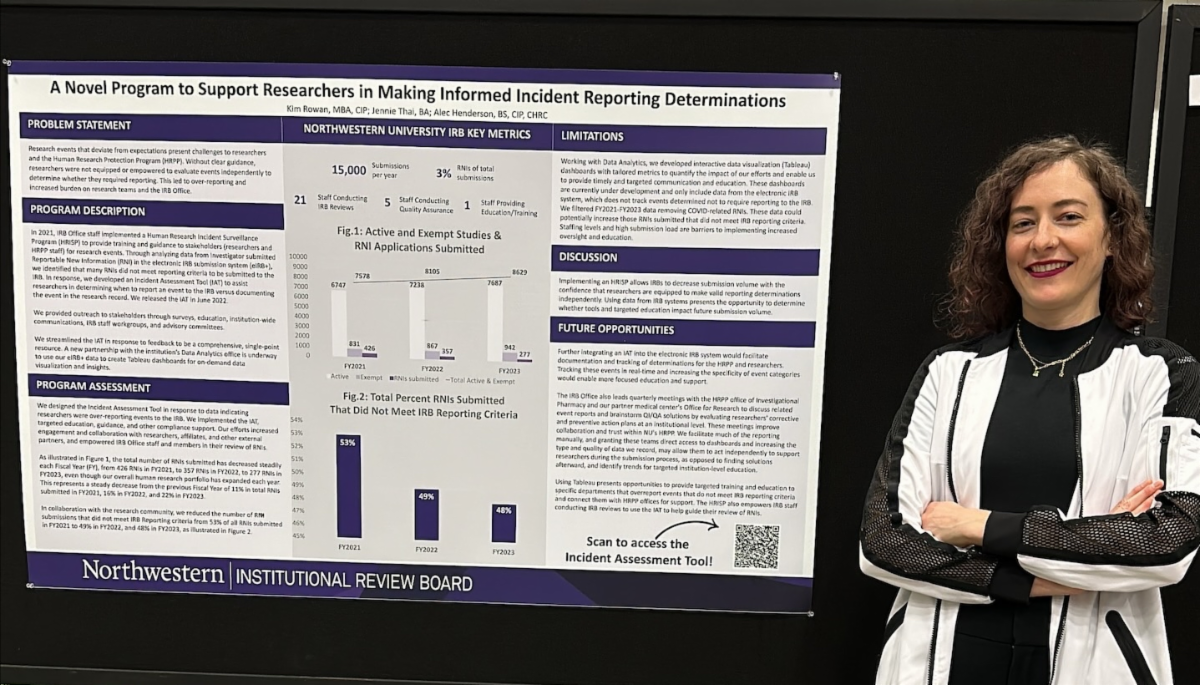
Representing the category of Flexibility and Innovation in Research Oversight, the poster evidenced that the IRB Office, in collaboration with the research community, reduced the number of investigator-submitted Reportable New Information (RNI) submissions that did not meet IRB reporting criteria. If you have any feedback or questions on this initiative or the IAT, reach out to IRBCompliance@northwestern.edu.
Fostering Accessibility and Inclusivity in Research
The IRB Office’s Fostering Accessibility and Inclusivity in Research (FAIR) Initiative, in collaboration with the Office for Research Diversity, Equity, and Inclusion (DEI) Office, would like to inform you of a grant opportunity from Center for Native American and Indigenous Research (CNAIR). CNAIR seeks to support those whose research/scholarship engages Native American & Indigenous communities and/or who center Indigenous research and Native American & Indigenous Studies (NAIS) methodologies. Applications are due January 12th. More information can be found here.
eIRB+ Test System for Researchers
For training purposes, the IRB Office is sharing the link to the test eIRB+ system where individuals can log into a mirror test system to learn how to navigate eIRB+ before working in the production system. Further instructions are on the IRB Office eIRB+ webpage.
UPDATED DOCUMENTS
The following updated protocol template is now available on the Protocol Templates and Form Page. Download the current version from the IRB Office website prior to use.
- Biomedical Protocol Template (HRP-593)
The following updated templates are now available on the Biomedical & Social Behavioral Consent Templates Page. See the IRB Office website for the tracked changes version of the Biomedical Consent Document (HRP-592), and please download the current version from the IRB page prior to use.
- Biomedical Consent (HRP-592)
- Social Behavioral Consent (HRP-582)
- Social Behavioral Consent & HIPAA (HRP-1721)
- Emergency Use (HRP-506)
- Verbal Consent (HRP-1710)
- Parent Consent & Permission with Child Consent (HRP-1711)
- Debriefing Information (HRP-1726)
The following updated checklist is now available on the Checklists and Worksheets Page:
- Prisoners (HRP-415)
The following updated SOPs are now available on the SOPs Page:
- Lapse (Expiration) of IRB Approval (Continuation of Current Participants) (HRP-063)
- Suspension or Termination Issued Outside of Convened IRB (HRP-026)
- IRB Meeting Conduct (HRP-041)
- Non-Committee Review Conduct (HRP-032)
The following Guidance is now available on the Policy and Guidance Page:
- Study-Specific Emergency-Disaster Risk Mitigation Planning (HRP-108)
STAFF EVENT SPOTLIGHT
IRB Takes DC!
We are excited to share that several of our IRB Office staff recently attended and presented at the 2023 Public Responsibility in Medicine and Research (PRIM&R) Annual Conference in Washington, D.C. Presentation titles and authors are listed below. The IRB Office is proud to have a staff base so deeply committed to the rights and welfare of participants in research and engaged in their own professional development while actively participating in the evolving human research landscape as leaders in research ethics and oversight.

Real-World Discussion Regarding How to Effectively Run an HRPP/IRB Office - Nathalia Henry Whitely, IRB Executive Director
IRB Closet Clean-Out: Keep, Toss, TBD - Piper Hawkins-Green, Associate Director, IRB Compliance and Reliance
Novel and Innovative Approaches on Inter-Institutional Agreements - Marcella Cooks, IRB Reliance Manager
Do You Really Want to Be the Single IRB (sIRB)? Navigating Risk Assessments When Requested to Sere as the sIRB for Federal Proposals -Monica Kane, IRB Reliance Analyst Lead