Study Team Members in eIRB+
Modifications that involve only the addition or removal of internal study team members, with up-to-date human research protections training, will receive automatic IRB approval upon submission.
Changing Study Team Members and Co-Investigators
To update the "Study Team Members" section, create a new Modification and select "Study Team Member Information" for the modification scope.
Reminders
- Personnel changes (e.g. PI change) may also require changing other study documents housed in eIRB+, including the protocol, consent document, and recruitment materials. In such cases, these documents should also be revised during the modification process by selecting “Other parts of the study” for the modification scope.
- Adding personnel to the Study Team Member list will not give that individual access to make changes to the eIRB+ study or receive eIRB+ notifications regarding the study. A person must be added to the Study Access List with “Edit” privileges to be able to make changes to the study. Only the PI and Primary Contact receive eIRB+ notifications regarding the study.
- Personnel should be added as either a Study Team Member or a Co-Investigator.
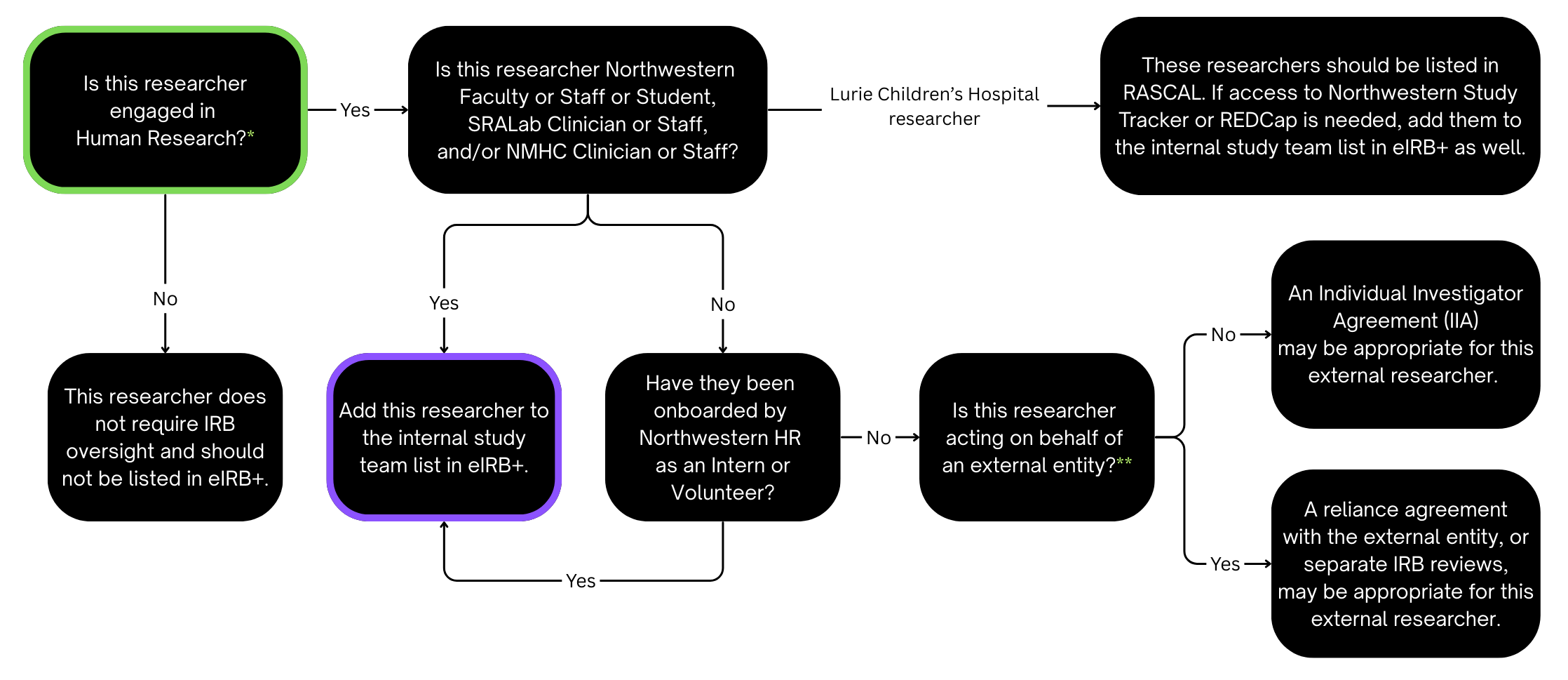
- Individuals who are not engaged in human research (see the Worksheet: Engagement Determination (HRP-311)) should not be added to the Study Team Members page. For example:
- Regulatory coordinators who do not interact with participants nor have access to identifiable participant data or biospecimens.
- Individuals who perform professional services that they typically perform for non-research purposes and do not administer any study intervention.
- Individuals who perform data or biospecimen analyses but only have access to de-identified data and biospecimens.
Changing the Principal Investigator
To change the Principal Investigator (PI) , create a new Modification and select “Other parts of the study” for the modification scope. This change must be made in the section of the eIRB+ application titled “Basic Information.” Additional requirements for changing the PI, including what must be included in the modification, are described in the Principal Investigator Transfer of Responsibility Guidelines.
Reminders
- The PI should not be listed in the Study Team Members section.
- Changing the PI may also require changing other study documents housed in eIRB+, including the protocol, consent document, and recruitment materials. In such cases, these documents should also be revised during the modification process by selecting “Other parts of the study” for the modification scope.
- Changing the PI for exempt research requires submitting a new study application instead of a modification. Review Updates to Exempt Studies for more information.
- Once IRB approval is granted, the new Principal Investigator (PI) automatically receives access to create, edit, and submit submissions and clarification requests, and will begin receiving all eIRB+ communications from the IRB.
Changing the Guest List (Study Access List)
Changes to the Primary Contact listed on the Guest List (Study Access List), or to individuals' Study Submission Rights, do not require a modification and may be updated at any time.
See eIRB+ Access Permissions for more information.
Interns and Volunteers
"Have they been onboarded by Northwestern or SRAlab HR as an Intern or Volunteer?"
If a researcher meets Northwestern's Unpaid Intern and Volunteer Criteria then please complete all corresponding forms and processes on the Hiring Unpaid Interns & Volunteers webpage. SRAlab has a similar process. After that process is complete they can use their netID in alignment with Northwestern policies for interns and volunteers. Once they have been onboarded as an intern or volunteer, complete the following steps to add them to the eIRB+ submission:
- Complete Human Research Protections Training for Northwestern University and Northwestern University-Affiliated Researchers.
- Register for eIRB+.
- Be added to the internal study team members list in eIRB+ by the Principal Investigator or designee.
External Study Team Members
"Is this researcher acting on behalf of an external entity?"
External study team members are either acting on behalf of an external entity or not, and this determines how they obtain IRB oversight. Either way, they should not have access to eIRB+. Actions in eIRB+ should only be carried out by an internal study team member.
Acting on Behalf of an External Entity
If a researcher does not meet Northwestern's criteria for interns and volunteers, and they are acting on behalf of an external entity, please review the following guidance:
- If they will receive IRB approval from another IRB (not Northwestern IRB), they should not be listed on the eIRB+ Study Team Members page.
- If they intend for their entity or institution to rely on Northwestern's IRB via a reliance agreement then review the Reliance Welcome Packet on the Northwestern Serving as the IRB of Record webpage.
Not Acting on Behalf of an External Entity
If an individual does not meet Northwestern's criteria for interns and volunteers, and they are not acting on behalf of an external entity or institution, an Individual Investigator Agreement (IIA) may be appropriate.
Reminders
- If a researcher is not engaged in human research, regardless if they are acting on behalf of an external entity or not, they should not be listed on the eIRB+ Study Team Members page.
- The Northwestern Office for Research advises on the process for "Research Visitor" appointments. These appointments often include the following titles: "Visiting Scholar", "Visiting Research Collaborator", "Visiting Pre-doctoral Fellow", or "Research Affiliate". More information can be found on the Northwestern Office for Research Research Visitors webpage.
FAQs
How do I register for eIRB+?
When should a study team member be removed from eIRB+?
If a study team member is no longer engaged in human research, or if they are leaving Northwestern, they should be removed from the study team list in eIRB+.
If Northwestern will serve as the IRB of Record for an external site, should I list all external study team members from that site?
If Northwestern is serving as the IRB of Record for an external site, only the site-PI or site responsible party should be listed in eIRB+ under external study team members. All other study team members at that external site, including their human research protections training and COI considerations, are the responsibility of that site to oversee.
Do external study team members with an Individual Investigator Agreement (IIA) need Human Research Protections Training?
If Northwestern will rely on an External IRB can I include external study team members in eIRB+?
If Northwestern is relying on an external IRB, only internal study team members should be included in eIRB+. Anyone external to Northwestern should work directly with the External IRB if they need to execute reliance agreements and be added to the study. Northwestern does not allow external sites to rely on us if we are relying on External IRB.
Does a Northwestern staff member need to be listed in eIRB+ if they are only helping with eIRB+ submissions (i.e. not engaged in human research)?
It's common for study teams to have personnel that support IRB submissions only. They do NOT need to be added to internal study team if they are not engaged in human research.
They can be given view and edit rights per study in eIRB+. See the eIRB+ Access Permissions webpage for step-by-step instructions.
Note: this is only for Northwestern faculty, students, staff, interns, and volunteers (with valid netID).
What should I do if the PI is unable to submit in eIRB+ but there is an urgent submission?
See the eIRB+ Access Permissions webpage for step-by-step instructions on giving "submit" privileges to another study team member. If a researcher has a Northwestern netID are they automatically an internal study team member?
Not necessarily. Having a Northwestern netID is not the same thing as having a appointment or employment at Northwestern.
Who do I contact if I'm having issues with my Northwestern netID?
The Northwestern IRB Office does not issue or manage netIDs. If the issue is with your netID please work with your department and/or Northwestern HR. If your netID is active but you're unable to access eIRB+ please submit an
eIRB+ Support Form.