Human Research Protections Training
Northwestern University requires all individuals involved in the conduct of human research to complete Human Research Protections Training and refresh their training every 3 years. The IRB will withhold approval of submissions if a member of the research team, including the Principal Investigator, does not have valid Human Research Protections Training. These requirements apply to all persons engaged in human research and listed on the eIRB+ study application. Those individuals include, but are not limited to:
- Principal Investigator and Co-investigators
- Individuals named on a study grant or contract proposal
- Individuals listed on an FDA form 1572 for the conduct of the research at Northwestern University or an affiliate institution
- Individuals who are responsible for the informed consent process or recruitment of research participants
- Individuals who obtain individually identifiable health information under a Northwestern Business Associate Agreement or Northwestern Individual Investigator Agreement.
All Principal Investigators (PIs) are required to maintain documentation (CITI Program-issued certificate) of Human Research Protections Training outside of the eIRB+ system for each study personnel engaged in the research. Please see Study Support Resources and Templates for the required documentation the PI must maintain on file.
Quick Links, Tips, and Best Practices:
- Collaborative Institutional Training Initiative (CITI) Program website
- eIRB+ webpage
- Human Research Protections Training Guidance (PDF)
- Northwestern University IRB Office requires and accepts only Human Research Protections Training; it does not require or accept Good Clinical Practice (GCP), Responsible Conduct of Research (RCR), Information Privacy and Security (IPS) or any training other than that specifically covering human research protections
- Training records will appear in eIRB+ after 24 hours. Please be patient, as training records will not appear in eIRB+ immediately following training completion in CITI due to systems integration cycles
- Contact the Department of Information Technology to reactivate an expired Northwestern NetID; temporary/returning researchers should avoid creating a new eIRB+ account with a new NetID as doing so often interrupts an important automated process and delays training and research timelines; see Troubleshooting below for more information
- Jump to training requirements for Northwestern University and Northwestern University-affiliated Researchers
- Jump to How to Transfer CITI Training
- Jump to Troubleshooting
Human Research Protections Training Pathways

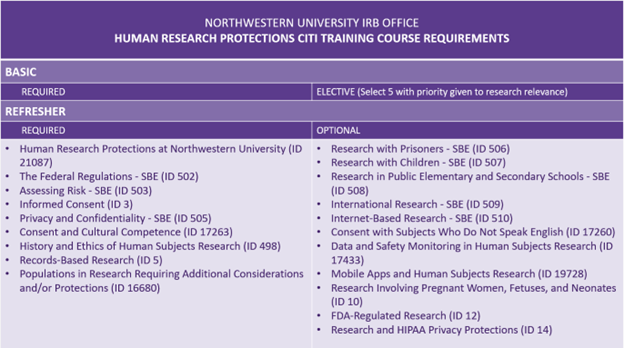
Human Research Protections Training Level Requirements
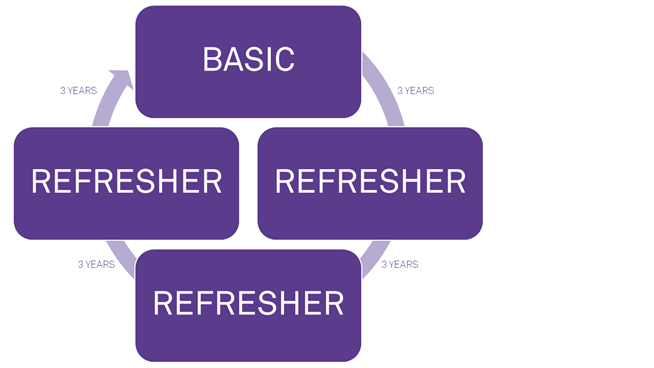
Human Research Protections Training Cycle
Human Research Protections Training Requirements by Group
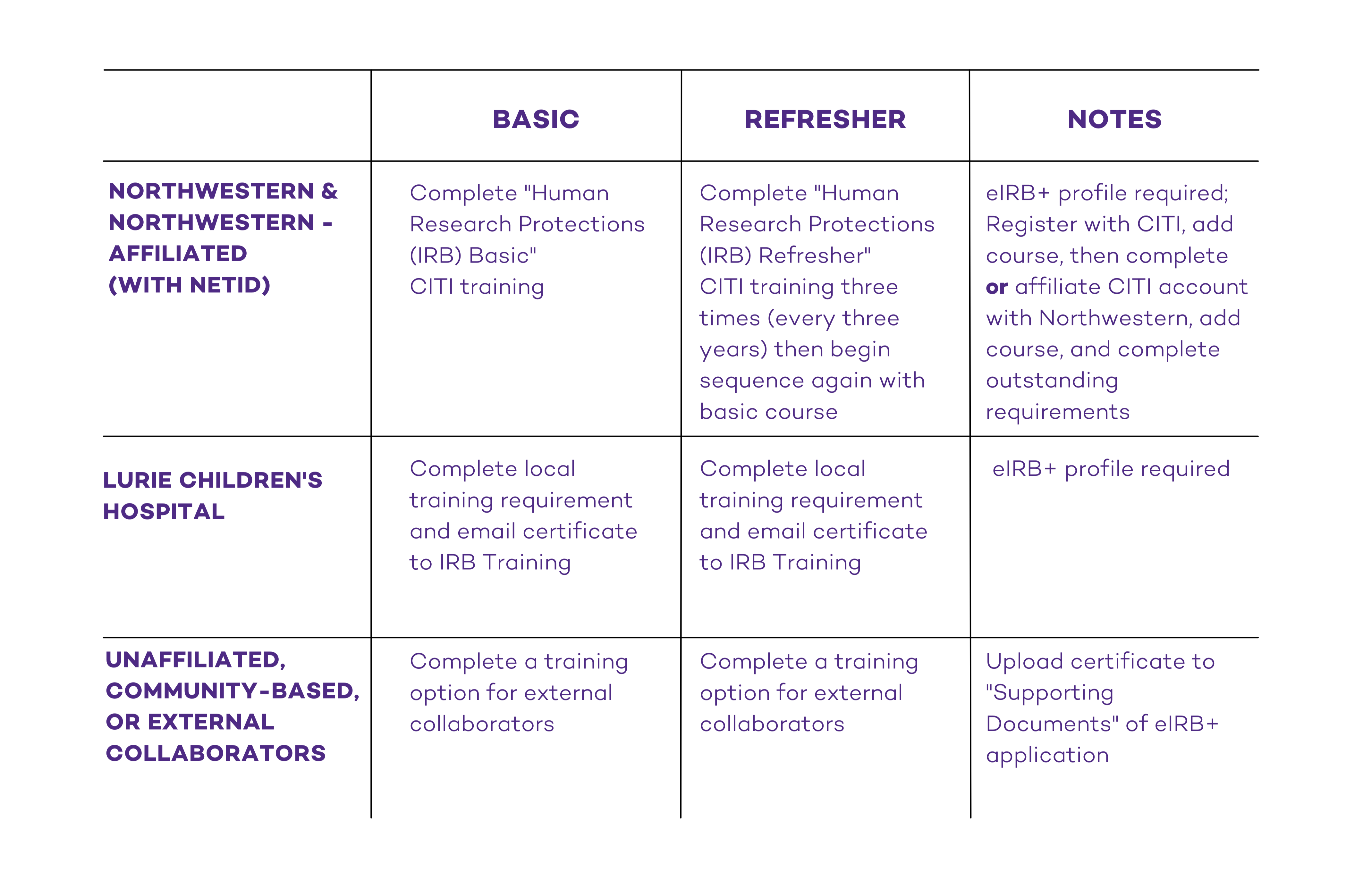
Northwestern University and Northwestern University-Affiliated Researchers
On September 1, 2023, training requirements moved from a dual-track (social behavioral and biomedical) to a single-track course for all Northwestern University and Northwestern University-affiliated human researchers. No action is needed, and current training records will continue to be valid until they expire. At expiration, the “Human Research Protections (IRB)” will be the only course enrollment option in CITI. Northwestern University-affiliated researchers include those at Shirley Ryan AbilityLab and Northwestern Memorial HealthCare (NMHC).
Ann & Robert H. Lurie Children's Hospital of Chicago
Unaffiliated, Community-Based, and External Collaborators
Government-Sponsored Research
Individuals conducting research sponsored by the U.S. Department of Defense or the U.S. Department of the Navy must complete Human Research Protections Training requirements and determine whether the study sponsor requires additional training. If the study sponsor requires additional training, email a copy of the completed training certificate to irbtraining@northwestern.edu for manual eIRB+ record entry.
Veterans Affairs researchers who have or will obtain a Northwestern NetID must register with eIRB+ and email their Human Research Protections Training certificate to irbtraining@northwestern.edu. Northwestern University IRB accepts only Human Research Protections Training.
Troubleshooting
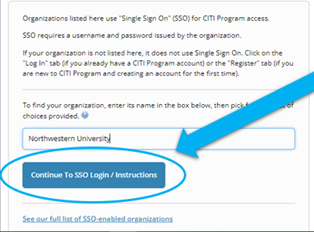
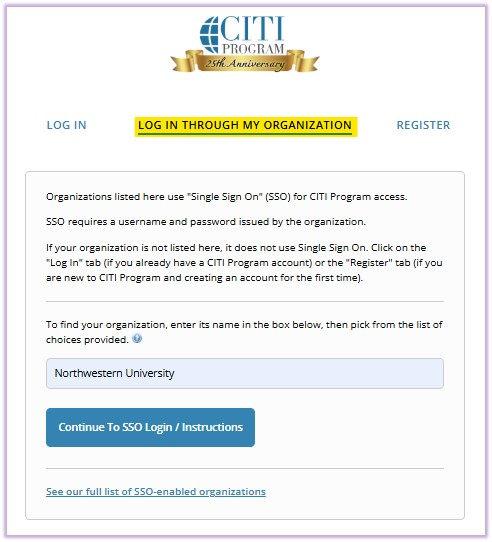
STEP 1: If the eIRB+ account does not reflect CITI training records 24 hours after registering a new CITI Program system user account or affiliating an existing account with Northwestern University AND completing the Human Research Protections (IRB) Basic course, likely, the user did not follow the instructions above. To resolve this issue, the user must update their CITI profile. This will resolve 95% of issues.
- "Log in through My Organization" > select the hyperlink "See our full list of SSO-enabled organizations" > search "Northwestern University" > select “Continue to SSO Login/Instructions” > enter organizational credentials (NetID), if prompted > when asked about existing CITI account, select “yes” to update CITI profile.
Please check the eIRB+ account for the training record after 24 hours following this process.
STEP 2: If the above steps do not resolve issues, the researcher likely has more than one CITI account, more than one eIRB+ account, or both.
To proceed, please include the following in an email to irbtraining@northwestern.edu:
- Subject line: “Request to check for multiple accounts”
- Message body: “The following researcher (state the individual's first and last name, and their current/active Northwestern University NetID) has taken all steps to complete training requirements and has logged into their CITI account using the Northwestern University SSO feature. Per Troubleshooting Step 2, please check for multiple eIRB+ and/or CITI accounts.”
NOTES: It is best practice to reactivate “expired” Northwestern NetIDs instead of obtaining new NetIDs to avoid issues with competing accounts. Contact the Northwestern University Department of Information Technology to reactivate an expired NetIDs.
The CITI Program Support website offers robust information on affiliating institutions, adding/enrolling in courses, and other actions required to integrate your CITI account with Northwestern records.
Submit an eIRB+ Support Form for further assistance.
 Users who already have a CITI Program account:
Users who already have a CITI Program account: